CLP Regulation. What you need to know about the CLP Regulation on Classification, Labeling, and Packaging of Substances and Mixtures.
CLP Regulation (EC) No. 1272/2008 is the Regulation on Classification, Labelling and Packaging of Substances and Mixtures. This regulation brings previous EU legislation on classification, labelling and packaging of chemicals in line with the GHS (Globally Harmonized System of Classification and Labelling of Chemicals). Its main objectives are to facilitate international trade in chemicals and to maintain the existing level of protection of human health and the environment.
GHS is the United Nations system for identifying hazardous chemicals and informing users about these hazards by means of standard symbols and phrases on package labels and SDSs (Safety Data Sheets), and it is continuously updated based on the GHS revisions published every two years. Since it is a Regulation, it must be applied in all Member States of the European Union without exception and without modification.
Globally Harmonized System - GHS
The GHS integrates safety information on hazardous chemicals globally by harmonizing classification criteria, labeling requirements, and guidelines for preparing safety data sheets.
The GHS is implemented and maintained by the United Nations to prevent discrepancies in information on chemical hazards worldwide. It is reviewed every two years.
Another goal is to streamline trade in chemicals: by applying the GHS to different countries, a chemical exporting company would not have to reclassify its products and re-label them to meet the requirements of the importing country.
However, these goals have not been achieved on a practical level, as the documentation needs to be adapted to each country's legislation.
GHS applies to:
-
All hazardous chemicals or mixtures.
GHS is not applicable:
- Pharmaceutical products.
- Food additives.
- Cosmetic products.
- Pesticide residues in food.
Any other questions?
Answers to Frequently Asked Questions
Its purpose is to guarantee a high degree of health and environmental protection as well as the free movement of substances, mixtures and objects.
One of the main purposes of the CLP Regulation is to determine whether a substance or mixture should be classified as dangerous, which is the starting point for hazard communication through labeling and safety data sheets (SDS) in accordance with Regulation (EC) No. 2020/878.
However, the regulation also serves other purposes:
- Regulate the packaging of chemicals classified as hazardous.
- To establish a harmonized classification of substances for the entire European Union.
- To create a classification and labeling list that includes all possible classifications of a substance
- To establish criteria for toxicology center notification (PCN).
- To create a unique formula identifier (UFI).
Like the Globally Harmonized System (GHS), it refers to three types of hazards for classification and labeling purposes:
- Physical hazards.
- Health hazards.
- Environmental hazards.
The different types of hazards are in turn subdivided into hazard classes and hazard categories.
Hazards need to be communicated to all participants in the supply chain: consumers, professional users and industrial users.
Two elements of hazard communication are used for this purpose
- Label.
- Safety Data Sheet (SDS).
However, the CLP Regulation does not regulate the content of the Safety Data Sheet, as this document is regulated by REACH and Regulation (EU) No. 2020/878.
Hazard communication
The label must be written in the official language(s) of the Member State(s) in which the substance or mixture is placed on the market.
The CLP Regulation sets out detailed criteria for labeling items:
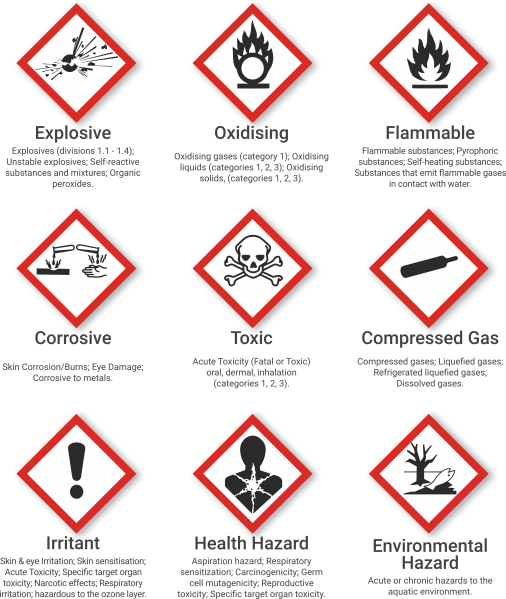
- Pictograms.
- Keywords.
- Hazard statements (H-phrases).
- Precautions (P-phrases).
- Additional information (EUH approvals).
- Product and supplier identification.
Similarly, the label must also include information specified in other laws, such as:
Volatile organic compounds (Directive 2004/42 / EC and amendments).
Labeling of detergents (Regulation (EC) No 648/2004 and amendments).
In the European Union, requirements for the preparation of safety data sheets in terms of format, content and other information requirements are established by Regulation (EC) 2020/878 amending Annex II of Regulation (EC) No. 1907/2006 (REACH).
In the vast majority of the world, the format of safety data sheets is divided into sixteen clearly differentiated sections that must contain all legally required information.
The safety data sheet must be:
- Written clearly and concisely so that it is understandable to the user.
- Adapted to the specific laws of the country and written in the official language of the country in which the goods are sold.
- The length of the safety data sheet is not regulated and must correspond to the hazard of the substance or mixture according to the information available.
No, they are different! Each country has its own national legislation, which affects both the configuration of the safety data sheet itself and the classification of the product.
All the particularities of each country must be taken into account, such as:
- Exposure Limit Values (OEL) for each country.
- The domestic safety and environmental legislation of the particular country.
- Inventory numbers of substances.
Safety Data Sheets (SDS) must be produced by companies selling chemicals or mixtures, whether they are a manufacturer, exporter-importer, distributor, trader or seller, and they must provide them free of charge with the first delivery of the chemical or when changes are made to the SDS.
The SDS needs to be updated if:
New information is received that affects risk management measures.
Changes are made to classifications.
Changes have been made to transport classifications.
Changes have been made to physical and chemical properties.
Other changes have been made that may have an impact on the classification of a substance.
Product composition has changed.
There has been a change in legislation.